Researchers from the Florida campus of The Scripps Research Institute (TRSI) have devised what they believe is a new and more efficient way to convert the major components of natural gas into useable fuels and chemicals. The research, led by TSRI Professor Roy Periana, uses chemistry and nontraditional materials to turn natural gas into liquid products at much lower temperatures than conventional methods.
“We uncovered a whole new class of inexpensive metals that allows us to process methane and the other alkanes contained in natural gas, ethane and propane, at about 180 degrees centigrade or lower, instead of the more than 500oC used in current 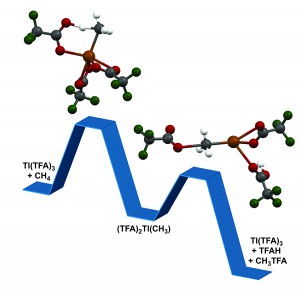 processes,” said Periana. “This creates the potential to produce fuels and chemicals at an extraordinarily lower cost.”
processes,” said Periana. “This creates the potential to produce fuels and chemicals at an extraordinarily lower cost.”
Methane, the most abundant compound in natural gas, is difficult and costly to convert into useable liquid products. With a need for lower carbon fuels, new processes are required to convert methane to fuel and chemicals in a way that is competitive with petroleum-based products.
Methane, ethane and propane, the major components in natural gas, belong to a class of molecules named alkanes that are the simplest hydrocarbons and one of the most abundant, cleanest sources of energy and materials. At the core of technologies for converting the alkanes in natural gas is the chemistry of the carbon-hydrogen. Because of the high strength of these bonds, current processes for converting these alkanes employ high temperatures (more than 500oC) that lead to high costs, high emissions and lower efficiencies.
Periana has been thinking about this type of problem for decades and has designed some of the most efficient systems for alkane conversion that operate at lower temperatures. However, when Periana and his team examined these first-generation systems they realized that the precious metals they used, such as platinum, palladium, rhodium, gold, were both too expensive and rare for widespread use.
“What we wanted were elements that are more abundant and much less expensive that can carry out the same chemistry under more practical conditions,” said Brian G. Hashiguchi, the first author of the study and a member of Periana’s lab. “We also wanted to find materials that could convert methane as well as the other major components in natural gas, ethane and propane.”Read More