Researchers have developed a new catalyst that could make ethanol-powered fuel cells feasible.
The research was done by a team of scientists at the U.S. Department of Energy’s Brookhaven National Laboratory, in collaboration with researchers from the University of Delaware and Yeshiva University, and was published online in the January 25 edition of Nature Materials.
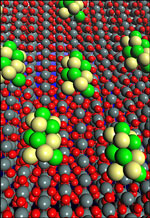 According to the researchers, the highly efficient catalyst performs two crucial, and previously unreachable steps needed to oxidize ethanol and produce clean energy in fuel cell reactions. Made of platinum and rhodium atoms on carbon-supported tin dioxide nanoparticles, the research team’s electrocatalyst is capable of breaking carbon bonds at room temperature and efficiently oxidizing ethanol into carbon dioxide as the main reaction product.
According to the researchers, the highly efficient catalyst performs two crucial, and previously unreachable steps needed to oxidize ethanol and produce clean energy in fuel cell reactions. Made of platinum and rhodium atoms on carbon-supported tin dioxide nanoparticles, the research team’s electrocatalyst is capable of breaking carbon bonds at room temperature and efficiently oxidizing ethanol into carbon dioxide as the main reaction product.
“Ethanol is one of the most ideal reactants for fuel cells,” said Brookhaven chemist Radoslav Adzic. “It’s easy to produce, renewable, nontoxic, relatively easy to transport, and it has a high energy density. In addition, with some alterations, we could reuse the infrastructure that’s currently in place to store and distribute gasoline.”
“The ability to split the carbon-carbon bond and generate CO2 at room temperature is a completely new feature of catalysis,” Adzic said. “There are no other catalysts that can achieve this at practical potentials.”

