The latest version of the U.S. Energy Information Administration’s “This Week in Petroleum” newsletter has a decided non-petroleum feel to it, as it leads off with an article on how to make biodiesel.
The article breaks down the process in six basic steps:
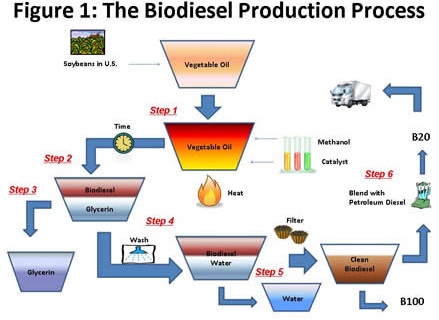
Step 1 is to heat the oil to a designated temperature and then mix it with the alcohol and the catalyst. The interaction between the heat, the oil, the catalyst, and the alcohol will create a liquid with two primary layers (Step 2). The top layer will be the biodiesel or fatty acid methyl ester (FAME). The bottom layer will be glycerin, a colorless and odorless non-toxic liquid that can be used for many different purposes, including pharmaceutical and cosmetic products. The reaction uses approximately a 10:1 oil-to-alcohol ratio input and yields 10:1 biodiesel-to-glycerin output. Commercial production processes typically use centrifuges to accelerate the separation process. Once the layers have separated, the glycerin layer is removed (Step 3). Then the biodiesel is mixed with water to allow impurities, including any remaining glycerin, to settle out (Step 4). Any excess methanol is removed through distillation or flash evaporation techniques. The biodiesel is then dried via application of heat to remove any remaining water and filtered (Step 5). The clean biodiesel is then ready to be used in its pure form, B100 (100 percent biodiesel), or in a blended form, such as B5 (5-percent biodiesel and 95-percent petroleum diesel) or B20 (Step 6).
Now, what they’re leaving out is that you’ve got to be careful when brewing up your own biodiesel. There have been some home fires from heaters that have malfunctioned, and the methanol used in the process is a hazardous material. It’s still a basically safe process that produces a good, clean fuel … you just have to be careful.

