Better methods for deconstructing biomass will lead to more efficient conversion to biofuels; however, this is one of the most complex processes in bioenergy technologies. Researchers at Oak Ridge National Laboratory (ORNL) have already uncovered information about how woody plants and waste biofuels can be converted more readily into biofuels. Now the team may have come one step closer to solving this riddle with the discovery of the chemical details behind the process.
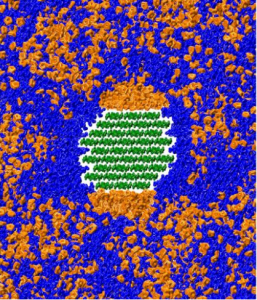
An illustration that demonstrates how THF (orange) and water (blue) phase separate on the surface of cellulose (green), thus facilitating its breakdown. Image credit: Barmak Mostofian
The research team is using computer simulations to study the chemistry of biomass deconstruction. Collaborators from the BioEnergy Science Center previously developed a pretreatment method for breaking down biomass that initiates delignification, the removal of the rigid plant molecule lignin. The cosolvent enhanced lignocellulose fractionation pretreatment involves aqueous solutions of tetrahydrofuran (THF), a versatile organic solvent. This cosolvent mixture uniquely interacts with cellulose, the main structural component of plant cell walls, to enable its breakdown.
Celluolose must be broken down in order to be converted into ethanol. Therefore if scientists can better understand how this process occurs, then they will be able to improve current pre-treatment methods or even find new solvents to boost the process.
As a means to discovery the chemicals involved in the breakdown of biomass, Smith’s team created models of up to 330,000 atoms and ran simulations on ORNL’s flagship supercomputer—the Cray XK7 Titan located at the Oak Ridge Leadership Computing Facility (OLCF). They found that the THF–water cosolvent phase separates on the faces of the crystalline cellulose fiber. These faces are distinct regions with which certain enzymes or molecules can interact. During the phase separation, THF preferentially binds to the hydrophobic, or “water-fearing,” faces of cellulose, and water preferentially binds to the hydrophilic, or “water-loving,” faces. THF enhances the binding of water molecules to the bonds that link two sugar molecules, which can potentially increase hydrolysis, the chemical breakdown of cellulose by water.
“We saw this phase separation, and we knew it might mean there was chemistry that was taking place on the surface that we hadn’t observed, that we hadn’t considered before,” said Micholas Smith, a CMB postdoctoral researcher.
The team also discovered that when they broke the cellulose apart, single chains of cellulose became surrounded primarily by water, while THF—because of its molecular structure—remained bound to the hydrophobic surfaces of cellulose. These results provided researchers with a fine-tuned understanding of the chemical properties behind the deconstruction of lignocellulosic biomass found in plants such as cornstalks, straw and other woody biomass.
“This information will help us find the minimum number of things we need to calculate to tell if a solvent is good for lignocellulose,” said Smith. “Hopefully, we will eventually be able to write a program that creates a better screening process for solvents and automatically selects the best ones.” In other words, the knowledge will help them identify new cosolvents.
The finding represents a first step toward determining the full pathway the THF–water cosolvent takes to break down cellulose. “Now we are limited to looking at the two end states of the cellulose deconstruction process,” added CMB researcher Xiaolin Cheng. “If we can map out the full pathway, that will be more relevant. In the future, with more computing power, we will be able to simulate the degradation pathway of the lignocellulosic biomass to understand what happens between the two endpoints.”

